islets transplantation
Beta-cell replacement therapy is currently seen as a potential cure to diabetes. Pancreatic islets transplantation has been proposed as a possible therapy to successfully replace beta-cells. However, this strategy has several countersides:
- complications related to immunosuppression
- overall decay of graft function in all patients within 1-5 years;
- shortage of donors: as at least two donors are needed for each receptor.
our strategy
The starting material is a highly innovative and versatile family of protein-based biopolymers. It will be combined with cutting-edge technologies to form a coating that will provide a physiologically ideal environment for the implanted cells so that they can survive and function in the body without being identified as hostile.
Once implanted, the final structure will be safe and biocompatible, semipermeable and strategically assembled to allow insulin diffusion from the inside, but also the access to blood supplies and nutrient flows to the transplanted cells.
Despite their efficiently immunoisolating their content, the coating will be able to promote complete integration and fusion of the capsule and the surrounding tissues generating a real continuity between the inner and the outer cells.
The challenge consists in designing a capsule that ensures the appropriate interaction between trasplanted pancreatic cells and the host tissues.
our challenges
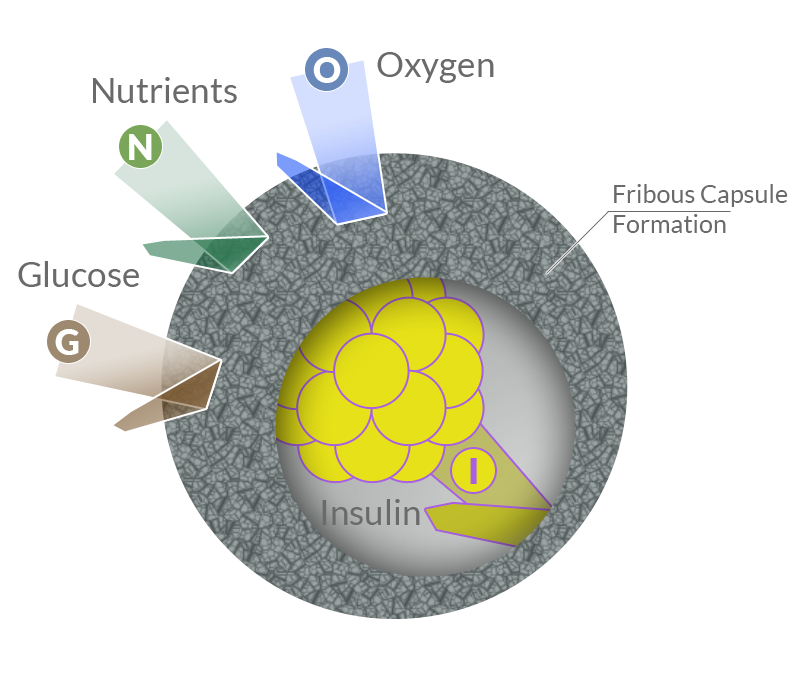
Biocompatibility
If the biomaterial is recognized by the immune system as foreign, acute and chronic inflammatory phenomena may occur and may give rise to a fibrotic process. The formation of a fibrotic capsule around the implanted pancreatic cells would drastically reduce the permeability of the implant surface and, consequently, thwart insulin release and cause death of implanted cells by hypoxia.
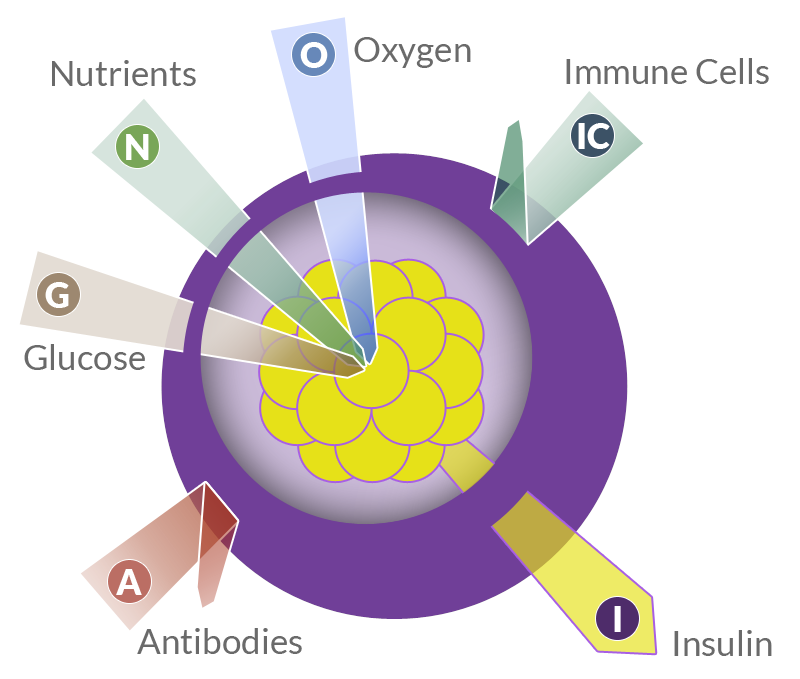
Capsule perm-selectivity
Semi-permeable nature and adequate selectivity. That means that the membrane must be permeable to the entry of oxygen, nutrients and glucose into the capsule and to the insulin output, but it must also be impermeable to immune cells, antibodies and, ideally, to potentially toxic cytokines and complement proteins.
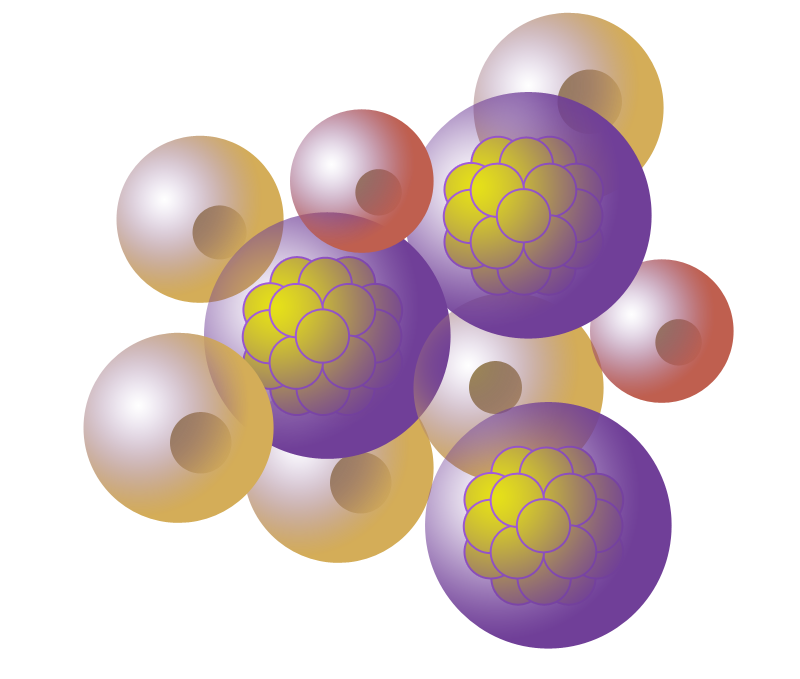
Capsule-host tissue integration
The surrounding cells from the host tissues must be able to interact with the implant, adhere to it and integrate it as a part of its own. In the short term, the implant must evolve from a discrete entity to an integrated cell niche that keeps its singularity but is connected to the rest of the organism.
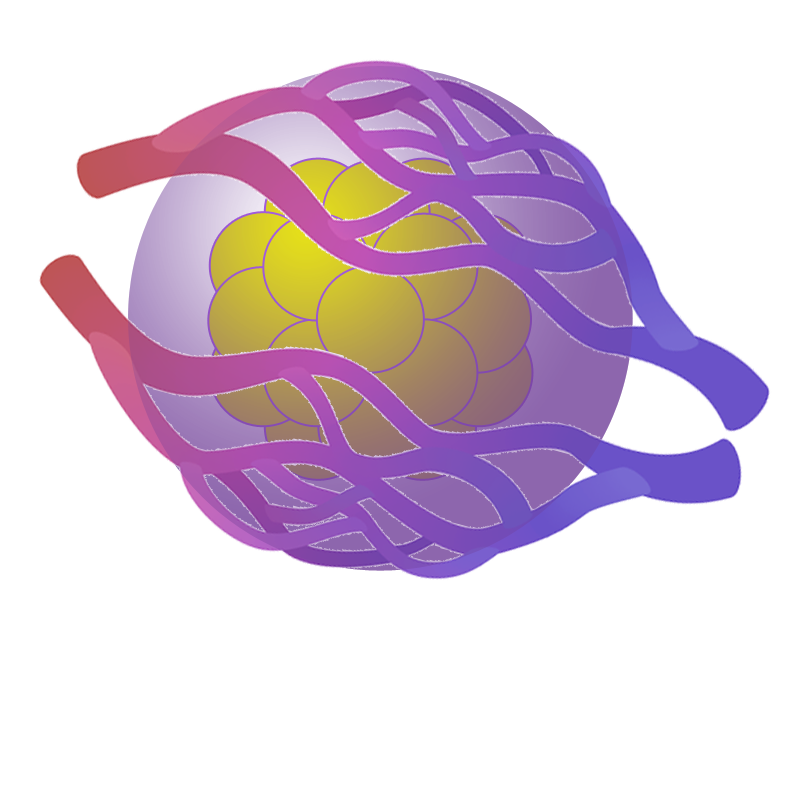
Angiogenesis
That is a specialized capsule-host tissue integration. The rapid vascularization of the implant and, as a consequence, the easy and rapid access of the implanted cells to the bloodstream improve the cell survival and accelerate the kinetics of exchange of nutrients, glucose and insulin between the implant and the bloodstream.